Scale PAAB Training Across Your Organization
The Enterprise Solution helps level-set Code knowledge across your business: Onboard teams quickly, track progress in real-time and secure preferred volume rates.
“Moving to the Enterprise plan gave us the visibility we were missing. We no longer rely on spreadsheets to track who is trained - the dashboard handles it all, and our new hires are onboarded instantly.”
Paul Costea, Systems Process Analyst, Roche

The Enterprise Advantage
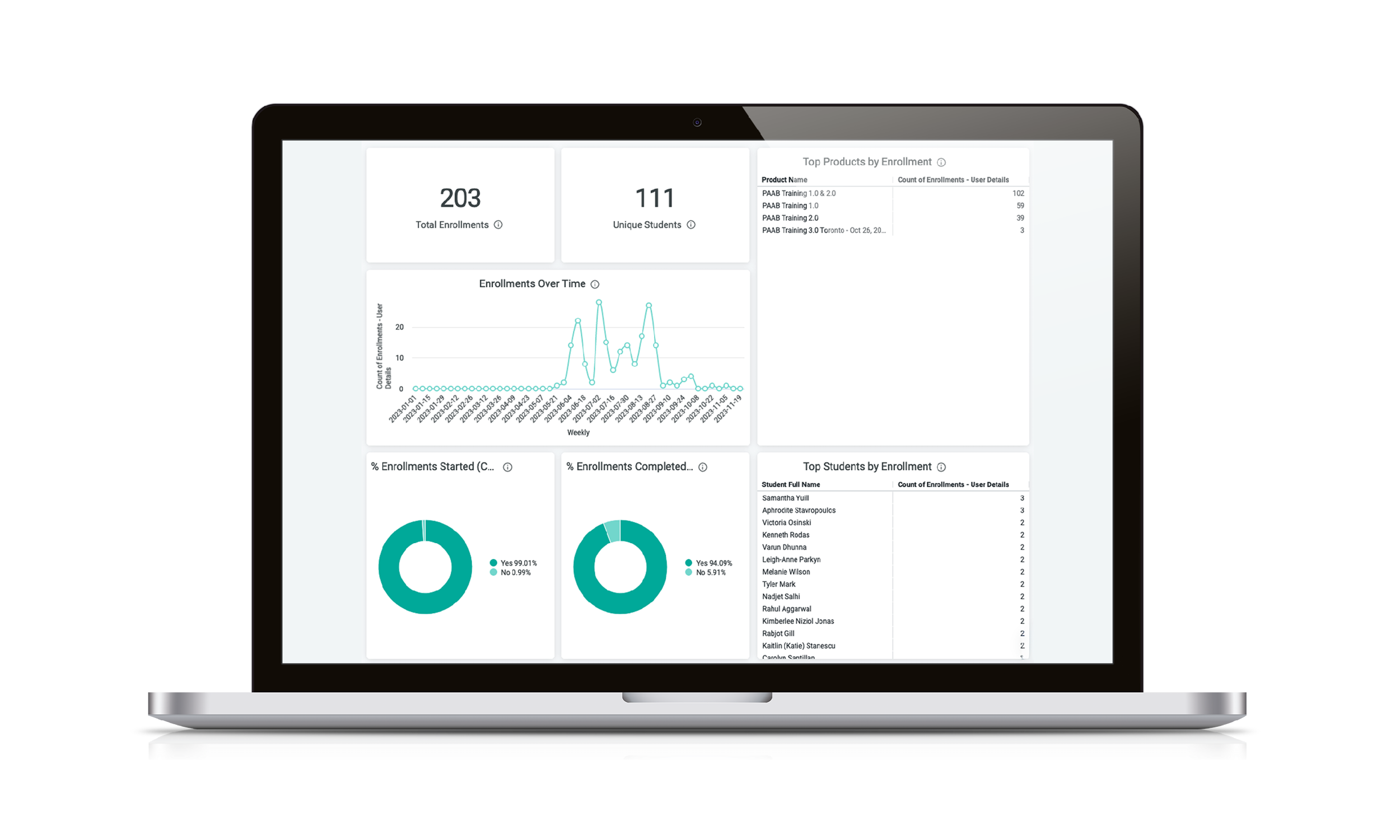
Full Visibility with the Student Dashboard
Stop guessing who is compliant. Get an at-a-glance view of who has started, is in progress, or has finished their training. Perfect for internal audits and ensuring 100% team readiness.
Dedicated Success Manager
You aren’t doing this alone. You get a dedicated Customer Success Manager (CSM) to help cocreate your internal communications plan. We provide step-by-step onboarding guides to drive adoption and ensure high completion rates.


Centralized Billing & Preferred Rates
Eliminate the hassle of individual expense reports. Cover all training costs with a single Purchase Order.
Enterprise partners unlock significant savings on both eLearning licenses and National Workshop tickets.
Choose the Path that Fits Your Scale
Standard Plan
Enterprise Solution
Best For
Individuals & Small Teams
Organizations & Large Depts
Pricing Model
Pay-As-You-Go
Annual Access Fee + Volume Rates
License Cost
Standard Rate
Preferred Rates (20% savings)
Workshop Cost
Standard Rate
Preferred Rates (10% savings)
Course Access
12 Weeks
Up to 52 Weeks
Admin Dashboard


Custom Reports


Payment Method
Credit Card
PO / Centralized Invoicing
Note: All plans include LinkedIn badges & certificates for all course completions.
FAQs about Enterprise Access
How long does it take to go live?
Admin setup takes approximately 3 working days. Once set up, staff invitations are typically sent within 5 working days.
How is billing handled?
We streamline the process. You can pay via a single Purchase Order (PO), bank transfer, or corporate card. Full invoicing is provided for your finance team.
What if we hire new staff mid-year?
No problem. New joiners can be enrolled immediately at your preferred enterprise rates for up to 12 months.
Can we add our own company content to the training?
PAAB Training courses are standardized, but we can build bespoke courses combining PAAB modules with your internal policies for an additional cost. Ask your Success Manager for details.

